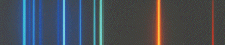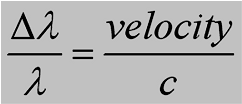Spectroscopy: A Key Part of the Astronomer's Toolbox
Key points: origin of emission and
absorption lines; spectra as a cosmic barcode; Doppler effect
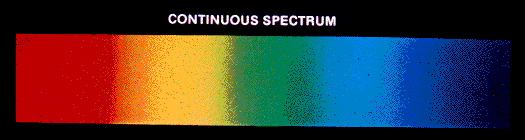
Spectrum: the distribution of intensities of light over
wavelength
In normal experience, solids and liquids tend to emit
blackbody spectra or spectra close to blackbodies. Gases tend to have more complex emission- and absorption-line spectra,
allowing us to learn a lot about their conditions.
Emission- and absorption-line spectra are produced by atoms (and molecules)
 |
Atoms consist of nuclei made of protons and neutrons, and electrons
around them. Hydrogen (1 proton) and helium (2 protons) are the simplest.
Most aspects of the behavior of an atom (e.g., in chemistry) depend just on the
number of protons and electrons; there are atoms
with up to about 100 protons, giving 100 elements, each with distinct
behavior. (figure by G. Rieke).
The electrons in an atom are held by the electric force, which is proportional to
1/r2 just like gravity. This force attracts positive and negative electric
charges, but repels like charges - two positives or two negatives. The protons in
the nucleus of the atom are held together by the "strong force", which is
clearly much stronger than the electric one but works only over very small distances. |
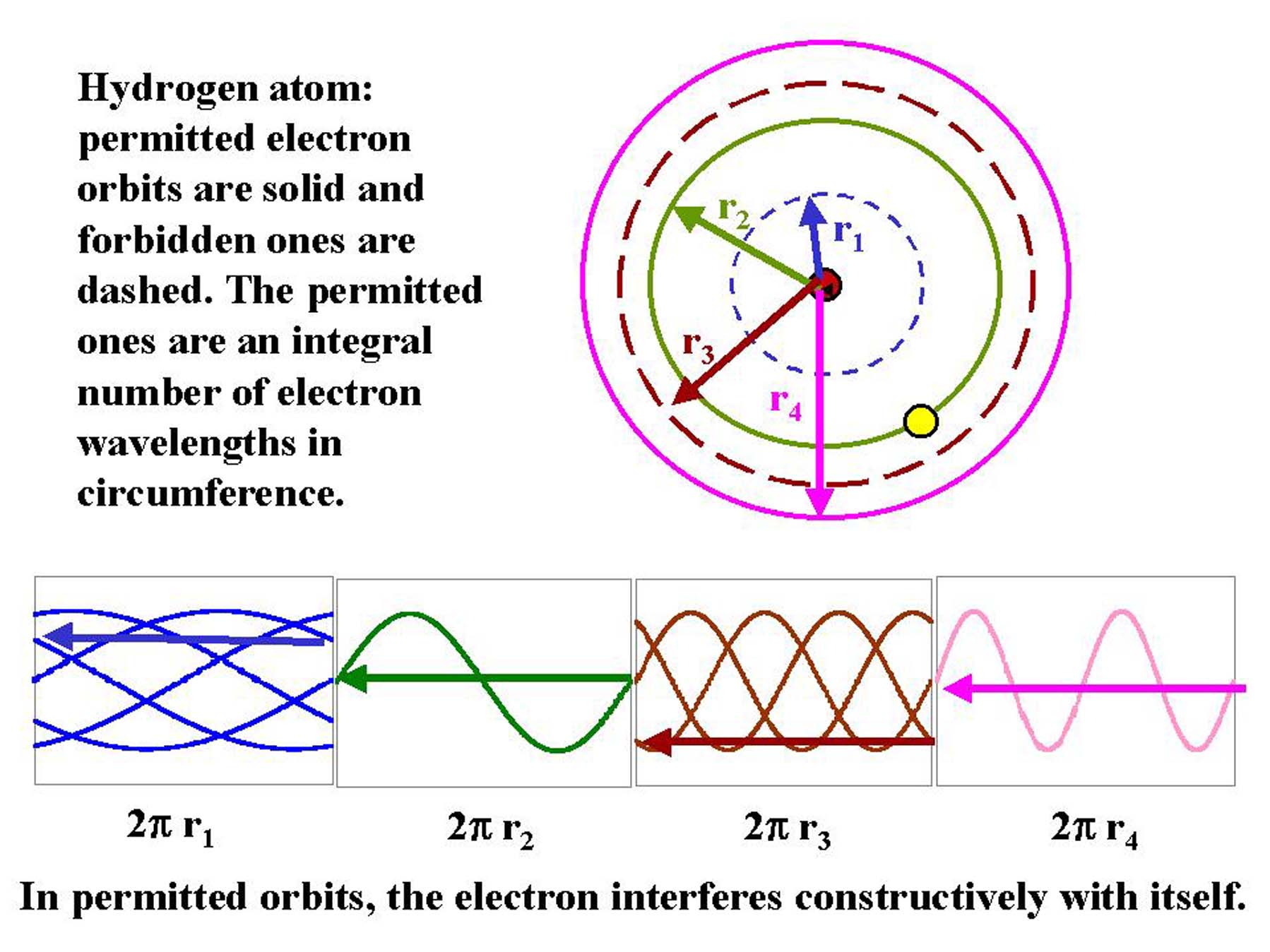 |
Electrons can only be in certain energy
levels in an atom because wave-particle duality means they interfere with
themselves in the other levels. This behavior is described by
the branch of physics called quantum mechanics.  (Figure by G. Rieke). (Figure by G. Rieke).
Electron transitions between energy levels lead to absorption or
emission of photons of specific energy corresponding to the energy level difference.
If an electron moves from an outer, higher energy orbit to an inner,
lower energy orbit, energy is released in the form of photon. The properties of this
photon depend on the energy difference between the orbits:
Energy = Eorbit 1 - Eorbit 2 = h = hc/ = hc/ |
 |
If a photon of exactly the right
energy "hits" an atom, it can be absorbed and cause an electron to jump to an
outer, higher energy orbit.(The Amazing World of Electrons
and Photons - Thinkquest http://library.thinkquest.org/16468/gather/english.htm)
A photon of the same energy is emitted when
the electron falls back down to its original orbit. |
 |
Electrons can also be raised to outer orbits when atoms collide
A photon of the characteristic energy is emitted when the electron falls back to
its original orbit. |
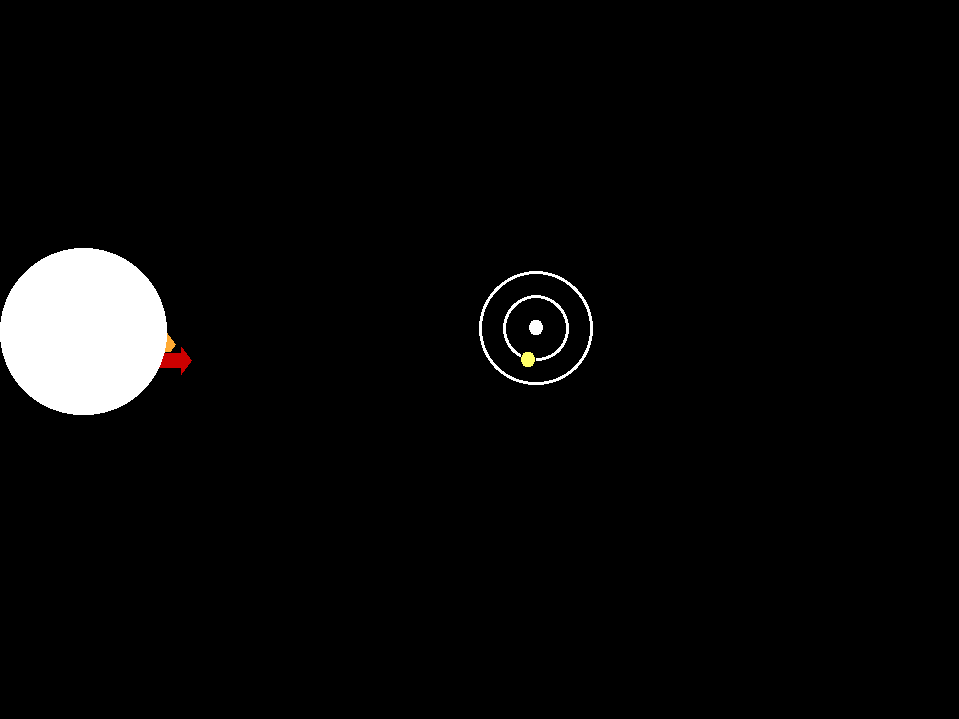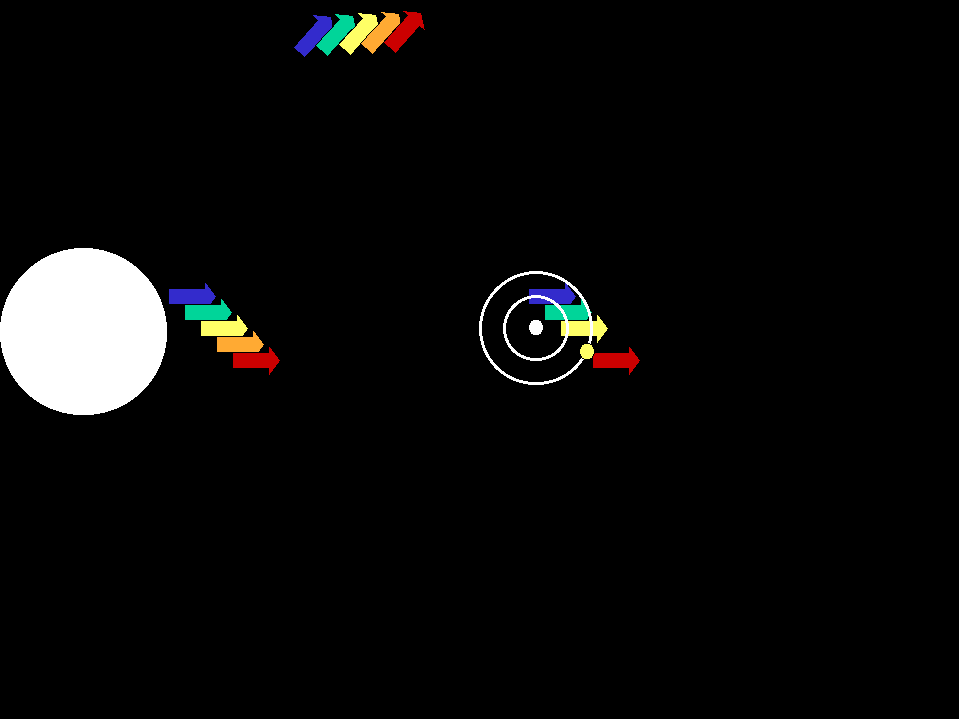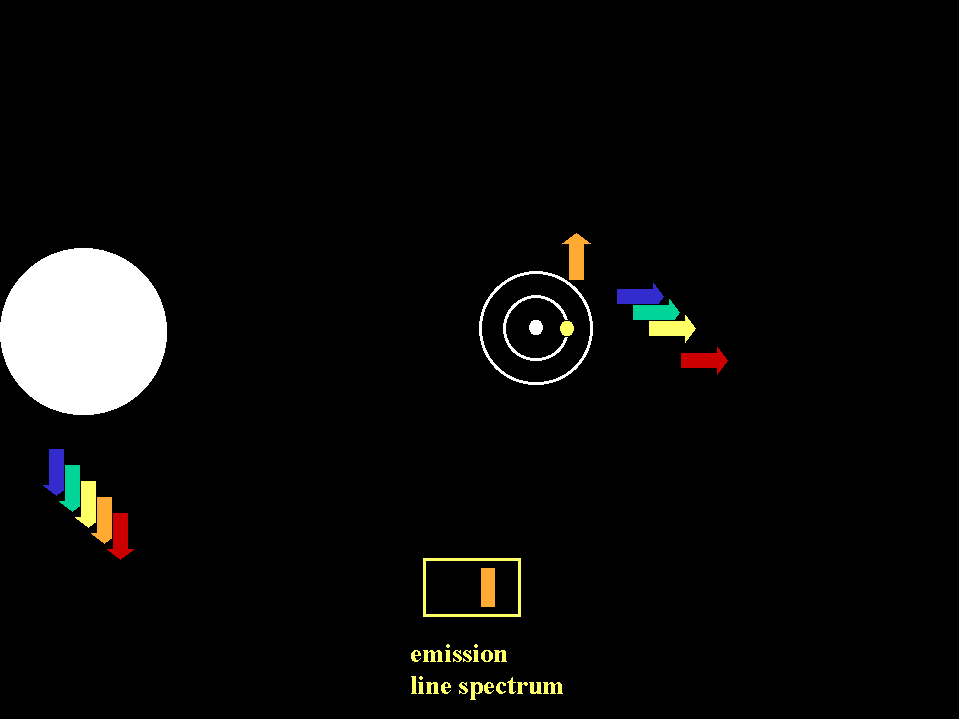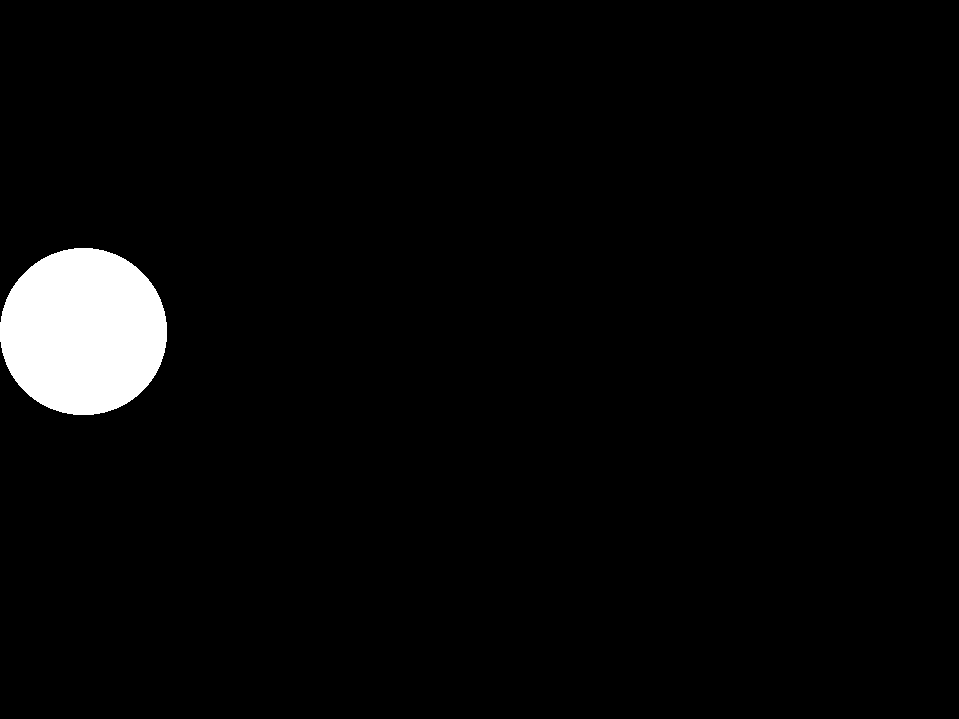 |
In astronomical situations, we may see either emission lines in a spectrum or absorption lines depending on the relationships of the the
sources and gases involved (animation by G. Rieke)
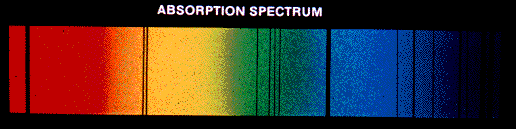
An absorption line spectrum is produced when cool gas lies between
a continuum source and us; the specific wavelengths absorbed by the atoms
in the gas are removed from the light that comes to us.
An emission line spectrum is produced when photons
are emitted by gas that is thin enough to be transparent in the continuum. |
Absorption- and emission-line spectra:
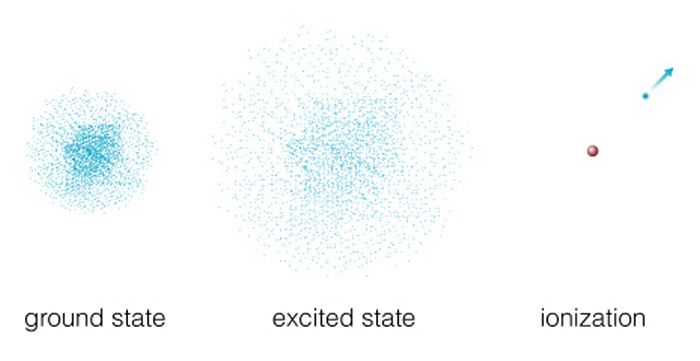
If even more energy is supplied to an electron, it can
escape from the atom leaving the positively charged nucleus. Because the electron is no
longer transitioning between two specific energy states, the atom can absorb a range of
energies in this situation. Electrons over a range of energies can be captured by the
positive nucleus, emitting photons over a range of energies
 |
Although it is convenient to draw protons, neutrons, and electrons as
little dots, quantum mechanics tells us that they cannot be located accurately and are in
fact more like fuzzy little fog clouds. We cannot predict precisely what they will do,
leading to a scientific confrontation with the philosophy of determinism: science shows
that there is fundamental uncertainty in what will happen in the future  (Figure from The Essential
Cosmic Perspective by Bennett et al.) (Figure from The Essential
Cosmic Perspective by Bennett et al.) |
Significance of Spectra
Spectroscopy of
astronomical sources has been a key to our understanding of the Universe because spectra
are:
1) Aids in determining temperatures (can be more reliable than looking at the wavelength peak)
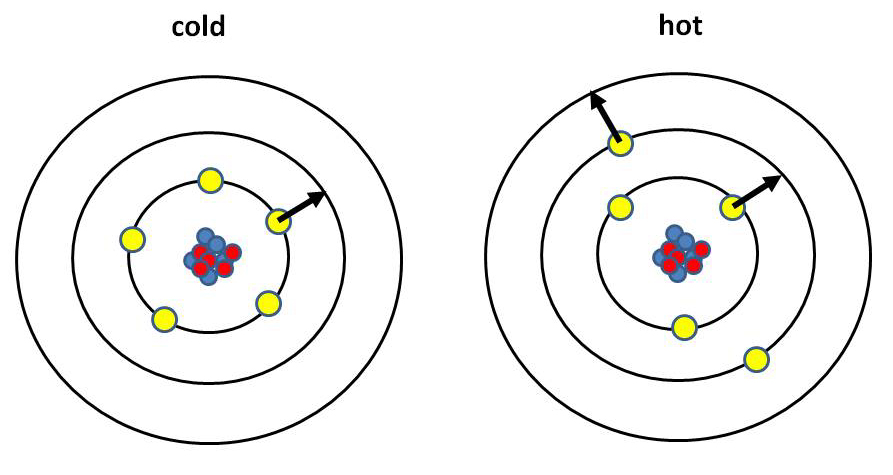 |
| The higher the temperature, the more electrons are in high energy orbits
or have escaped altogether from their atoms because high-temperature atoms run
into each other at high enough speeds to shift the electrons. As a result, in
hot gas there is emission and absorption of specific lines associated
only with the high energy orbits that are inaccessible at low temperature. In
this example, the cold atom can only absorb photons with energy
corresponding to the transition between its two lowest energy levels,
whereas the hot atom can absorb photons of that energy, plus ones
corresponding to the transition between its second and third levels. |
We can consider spectra to be a "cosmic
barcode" that identifies the conditions in the object (Fraunhofer spectrum of the sun, from R. Fosbury, http://www.stecf.org/~rfosbury/home/photography/Eclipse99/csp_description.html)

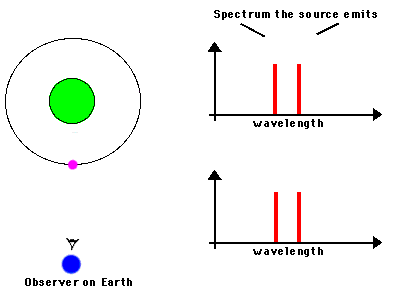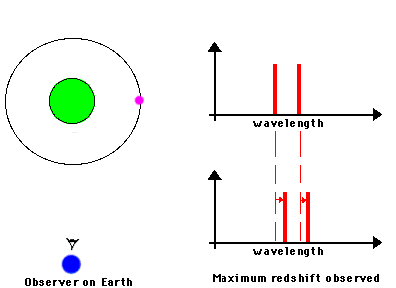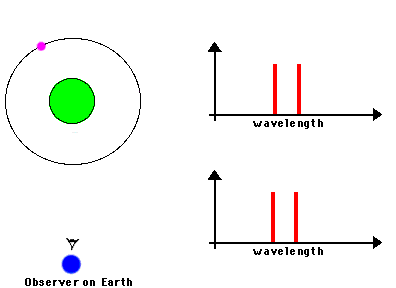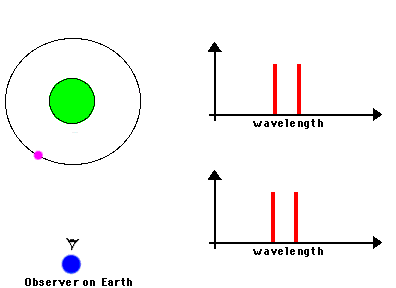
3) Indicators of motions. By understanding the Doppler effect, we can use spectra as a
means of measuring the speed at which a distant object is moving
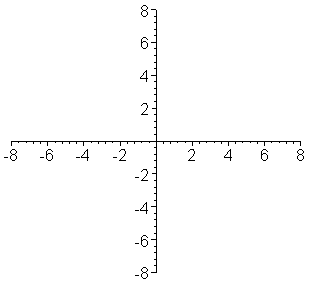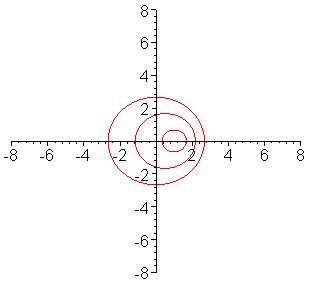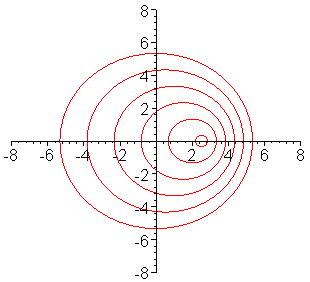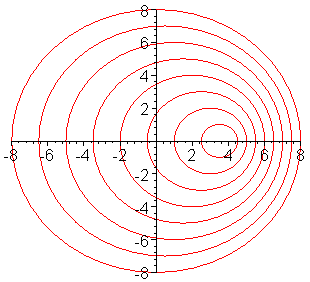 |
The frequency of a wave is modified by the motion of a
source toward or away from the observer. In the case of electromagnetic radiation:
Toward produces "blueshift" ==> spectral lines are shifted towards shorter wavelengths
Away produces "redshift" ==> spectral lines are shifted towards longer wavelengths
This animation shows why these changes occur. As the source moves toward the
right, it "catches up" with the waves it has emitted in that direction and
shortens their wavelength, shifting the light to the blue. Similarly, it "leaves
behind" the waves it has emitted to the left, shifting the light to the red. (From
Univ. of Saskatchewan, http://physics.usask.ca/~hirose/ep225/animation/doppler/anim-doppler.htm) |
 |
See how the wavelength of the sound into the boy's left ear is
shortened in wavelength because the ambulance is approaching him, while the wavelength of
the sound into his right ear is lengthened because the ambulance is moving away. The
Doppler effect with light is similar to that with sound. It is how the
policeman's radar gun works - it sends out a radio wave of a fixed
wavelength and then measures the Doppler change in wavelength when the wave
comes back, reflected off your car. The change in wavelength goes as the
speed you are driving toward the policeman.
(From
Japanese Aerospace Exploration Egency, JAXA, http://spaceinfo.jaxa.jp/note/shikumi/e/shi10_e.html.) |
Doppler Effect as a Speedometer
The amount of frequency (or wavelength) shift is proportional to an object's
velocity
where c = speed of light and the Greek capital delta (the triangle)
means the "the shift in" - that is the shift in wavelength divided by the original wavelength
is equal to the speed of the source divided by the speed of light.
Why are photons so important to astronomy
Test your understanding before going on