Quantum Mechanics
At the beginning of the 20th Century, in an effort to deliver a better lecture, Max Planck tried to derive the theory of blackbody radiation using a new assumption, that light energy came in packets of certain sizes. To his surprise, the packet derivation was actually better than those that had come before: it actually predicted the peak in the blackbody curve that we describe by the Wien Law. Previously, everyone knew that blackbodies emitted at this peak (Wien had shown it!), but the theory could not explain or even fit it. However, not long after, Einstein showed that the packetization of energy in light was actually the way it behaved, not a mathematical trick at all. The term "quanta" was used to describe these packets, and a new quantum theory to describe the behavior was developed over the next 30 years.
As quantum theory developed, it was found to require that not only photons, but electrons and protons - all particles of matter - had wave-particle duality. That is, the things that had been thought to be nice material particles - electrons, protons, neutrons, etc. - acted like waves under some conditions and interfered with themselves, just like light was known to do. And light, which was well known to act like a wave and interfere with itself all the time, could also act like a particle under the right circumstances.
Waves are not well located in space, so we should draw an atom with fuzzy clouds in place of little round balls for the electrons, and smaller clouds for the protons and neutrons in the nucleus. The cloud tells us where the proton, neutron, or electron is likely to be. If we can convert it or observe it as a particle, it is most likely to be observed at the thickest part of the cloud, and less likely but still possible to be observed in the thin parts. But already, this indicates that we cannot assign a precise position to the particle; all we have is a probability distribution telling us where it is more or less likely to be. Heisenberg in 1926 expanded this dilemma to a more general theorem, stating that it was fundamentally impossible to know exactly both members of various pairs of quantities about a particle simultaneously. An example is to know the position and velocity at the same time, exactly. Heisenberg pointed out that, to measure the position, we might shine light on the particle, but then the collision of the photons with it would change its velocity. The very act of measurement of one quantity had disturbed another, and because of the fuzzy nature of the light photons, we could not tell exactly what change they had made in the velocity of the particle.
All of this behavior is described mathematically in the Schroedinger equation, which calculates probabilities for the wave packets representing particles to behave in various ways. These packets are called wave functions. The Schroedinger equation makes it explicit that the wavelength of the wave function gets shorter in proportion to the mass of the particle. In principle, the wave function applies to anything, not just tiny fundamental particles of physics. However, massive objects like a house have such short wavelengths by this dependence on mass, that they can be located precisely - this behavior determines the transfer from the scale where quantum mechanics really has a big effect on how things behave (basic particles in physics), to that where Newtonian mechanics is a good description and things appear to be highly determinant. However, even on this large scale, the principle of the uncertainty predicted by quantum mechanics still remains. This subject is interesting in philosophy because it implies that there is a certain uncertainty in how things will behave, although physics tells us that the uncertainty is very small and we can ignore it in normal life.
Another discovery that was made along with the development of quantum mechanics is that each fundamental particle with mass in physics has an antiparticle, with the opposite electric charge. There is an anti-electron, the positron, and an anti proton, anti neutron, and so forth. Thus, we can have anti-hydrogen, with an anti-proton for its nucleus and an anti-electron circulating around it (in a properly poorly defined orbit as ordained by quantum mechanics). We can have, in fact, the entire collection of elements composed of anti-particles, and could build a whole Universe of them that would be virtually indistinguishable from the Universe we live in. However, when anti-particles combine with particles (say a proton with an anti-proton), they annihilate each other and their entire mass appears as energy, gamma rays. Thus, we would have to be careful to avoid touching anything from our anti-Universe, or there would be a loud explosion and a big hole left where it occurred.
An interesting aspect of quantum theory is that it envisions that particle/anti-particle pairs can materialize in empty space, and then recombine leaving empty space. By the uncertainty principle, this happens without a trace so long as the particles exist for a very short time, because our ability to say that space is empty is subject to uncertainty.
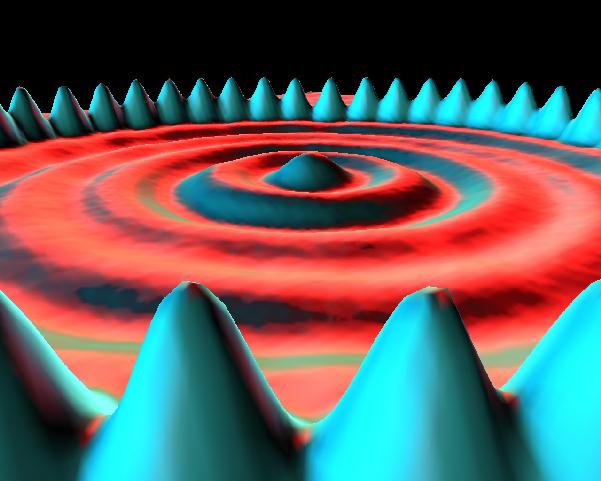
Image from IBM Scanning Tunneling Microscope Image Gallery, http://www.almaden.ibm.com/vis/stm/gallery.html. The image shows the ripply energy field set up inside a circular "corral" of 48 iron atoms.