
Formation of amino acids in the laboratory
| Once the meteor bombardment had settled down a bit, the early Earth
would have been largely covered with oceans, with occasional volcanic islands. The
atmosphere would have been warm and turbulent, with frequent violent storms. (Artist's
concept from NASA Origins Roadmap, 2003, http://origins.jpl.nasa.gov/habitable-planets/invest16.html)
|
 |
 |
Stanley Miller (then a graduate student working with H. Urey at the University of Chicago) tried to re-create conditions similar to the early earth in the laboratory. To the left, a much older Miller is shown with a re-creation of the experiment. (L. E. Orgel, http://www.geocities.com/CapeCanaveral/Lab/2948/orgel.html). |
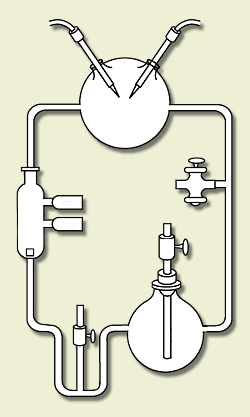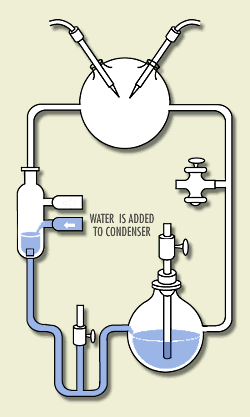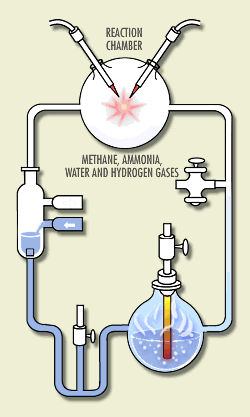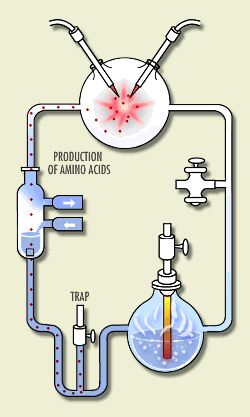 |
Here is a sketch of his apparatus, animated to show how
it worked. (S. Henehan, http://www.accessexcellence.org/WN/NM/miller.html) At the time, it was thought that the atmosphere was rich in the hydrogen-rich gases methane, ammonia, hydrogen, and water vapor. Miller put these chemicals in a closed system of flasks. He warmed a soup of these chemicals, circulated them through a region where they were subject to electric sparks (simulating lightning), and cooled them and returned the products to the soup. Within a few days, the soup was rich in amino acids, the building blocks for life -- 15% of the carbon was in these acids!
We no longer believe that the chemicals Miller used were the correct ones to simulate the early atmosphere, but his experiment introduced a new type of experiment - to try to synthesize the first steps toward life in the laboratory. |