 How Life Formed on the Earth
How Life Formed on the Earth How Life Formed on the Earth
How Life Formed on the Earth
Key points: Earliest life; critical steps toward life; RNA and DNA; evolution
Life probably couldn't have formed during the bombardment as the earth assembled from infalling asteroids.
 |
Early stages of formation of Earth; much of the surface is still
molten, by David Hardy http://www.hardyart.demon.co.uk/html/main.html
|
 |
A bit later, with a solidified surface but still the bombardment continues, by Don Dixon |
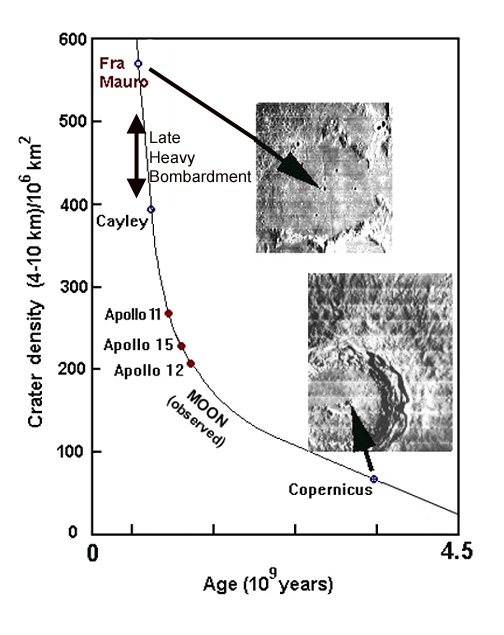 |
We can judge the rate from the cratering of the moon -- erosion has not erased all the traces of this episode there, as it has on the earth. The bombardment was very intense up to about 4 billion years ago. (illustration adapted by G. Rieke, after J. & C. Lunine, "Earth") In fact, there is evidence that an intense "aftershock" of large impacts resurfaced the moon around 3.9 billion years ago (the Late Heavy Bombardment). Just during that episode, the earth might have experienced as many as 1700 events powerful enough to leave craters larger than 20 km in diameter and capable of widespread havoc and extinction. |
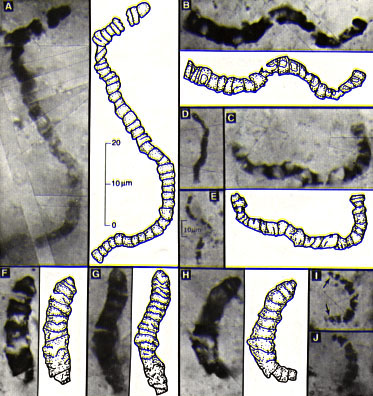 |
Despite this bombardment, very primitive life had already formed by 3.5 billion years ago. To the left are microbacteria fossils of this age (used as the standard against which to judge the claims for microbacteria in the meteorite from Mars). (J. W. Schopf, http://www.astrobiology.ucla.edu/) |
So, Life formed quickly! How?
 |
After the initial impact bombardment, the
surface of the earth must have quickly established conditions similar enough to the
current ones for life to form. The heat from assembly of the planet would result in much
more volcanic action, however, and typical volcanic gases would be expelled into the
atmosphere: carbon dioxide, carbon monoxide, nitrogen, water vapor. it is generally
believed that the convection in the mantle would be too strong for plate tectonics. Once
the water vapor had condensed into oceans, they would have covered most of the surface,
with vigorous volcano eruptions below the surface and occasionally a volcanic island
sticking above it.Ultraviolet radiation from the young sun was much more intense than now,
and the gases then in the atmosphere would have done little to shield the surface. Moon setting on early Earth, Don Dixon. |
A classic experiment in 1953 has dominated thinking about how amino acids
might have formed![]()
 |
Stanley Miller used a mixture of chemicals and electric sparks, and produced amino acids readily in the laboratory. (L. E. Orgel, http://www.geocities.com/CapeCanaveral/Lab/2948/orgel.html). Until recently, it was thought that the atmosphere was more likely to be dominated by gases with very different chemical behavior than those Miller used, and with these gases the experiment does not work. However, the results from our recent exploration of Titan show an atmosphere almost identical to the chemical mixture Miller used, so the whole topic is still under assessment. In any case, he set the stage for a whole series of experiments trying to establish the conditions for early life in the laboratory - and seeing what happens. |
In addition to the Miller experiment, it is possible to make amino acid in other ways, such as in volcano vents. More curiously, they are observed in interstellar clouds, and they have also been created in the laboratory with experiments that recreate the conditions in interstellar gas clouds where stars form. It is possible that they were created in this way external to Earth and brought to the planet in the rain of interstellar material that has continued (although diminished) from its birth to the present. Good! We seem to have a number of ways of getting started.
2.) Make molecules that can harness energy and replicate themselves
Amino acids are relatively simple. They have none of the aspects of life - they are just raw materials, like bricks for a skyscraper. Given the complexity of what had to happen next, it is not surprising that it has eluded us almost completely. It is possible (C. DeDuve, Vital Dust) that the next step was to harness energy, by building complex molecules that can store chemical energy and release it through chemical reactions (these reactions are called fermentation and, yes, the process that converts sugars to drinking alcohol is one of them). The other key to making life is to form molecules from amino acids that "replicate" themselves, that is, encourage the formation of identical molecules to themselves. As soon as we have molecules that harness energy and can make copies of themselves, we set in motion the "natural selection" engine. The molecules that survive well in a given environment will stay around to make more replicas of themselves, and will come to dominate the chemicals in that environment.
| Proteins - made of amino acids - are the main building material for
living organisms but cannot reproduce |
 |
The first very primitive organisms would have been too small and fragile to last for long. Although RNA is a very complex molecule, it had to be "invented" quickly to be successful at all. Nonetheless, it has proven difficult to produce primitive RNA through experiments analogous to Miller's, so our understanding of this key step is not very complete. One idea is that some predecessor to RNA that was simpler, but still able to replicate, must have been created first. For example, one proposal is that clays acted as a kind of template that produced RNA-like molecules - a possibility that seems to be supported by some laboratory experiments.
Once RNA had formed, we had life (albeit of a very primitive nature)!
RNA replication still has a fairly high error rate, perhaps 1%, which limits the
complexity of chains that can reproduce. A specialized molecule eventually developed, DNA,
which replicates far more accurately. The replication process with either RNA or DNA is an
incredible feat of organic chemistry. It is built around phosphate and sugar molecules,
that give DNA its structural "backbone", and four molecular appendages onto
sugars, nucleotides, that carry the information: adenine, cytosine, guanine, and thymine![]()
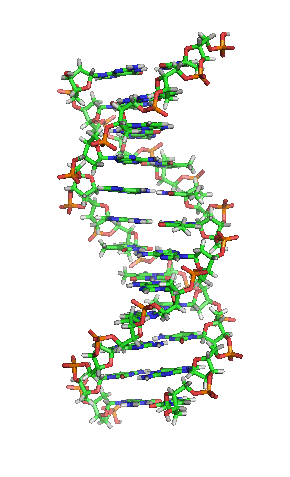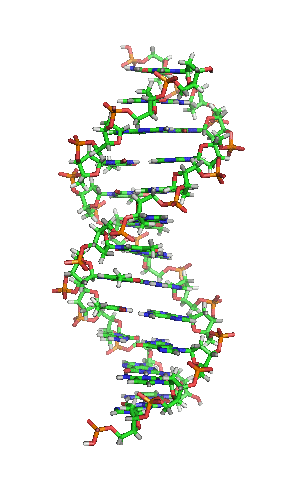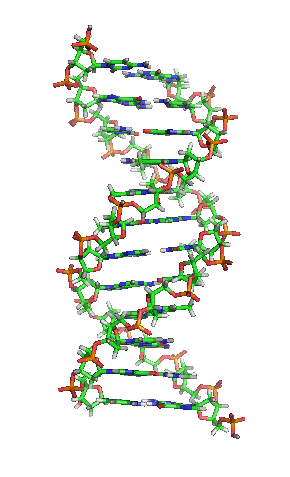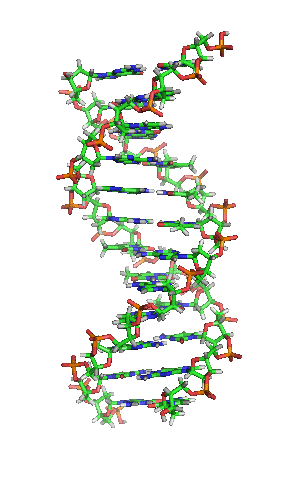 |
The famous "double helix" structure of DNA is a result of
the most efficient mechanical packing of the molecule. Far more interesting is that every
sequence of three nucleotides encodes instructions for making a unique protein molecule,
of the 20 proteins that are used as the building blocks of life. When scientists
"decoded" the human genome, they determined the string of proteins spelled out
by human DNA. (from Wikipedia commons
http://commons.wikimedia.org/wiki/File:DNA_orbit_animated.gif)
|
A string of DNA acts as a template for producing another string that is its
"negative", that is has the same encoding but with each nucleotide replaced with
its "complement": adenine for thymine and thymine for adenine, and cytonine for
guanine and guanine for cytonine. The process occurs in water and begins when the
complements are attracted to the DNA nucleotides by "polar" bonds. A polar bond
is created by the weak electrical asymmetries in a molecule, not by the normal process of
sharing electrons between atoms. The chemical reactions are assisted by a polymerase, a
molecule that moves along the DNA and somehow helps the new DNA assemble correctly. Once
the new DNA is complete, it can detach from the old one by the breaking of those polar
bonds - they act like a perforated line in a sheet of paper that allows a form to be torn
off after it has been filled out according to the instructions. The animation below gives
an idea of the whole process ![]()

The DNA in each cell of an organism contains the full set of instructions for making a new organism (with the important exception of sexual reproduction to be discussed soon). If the DNA in a human cell were laid out as a single string, it would be about as long as the height of the human and would contain about a billion bytes of information (in computer parlance).
3.) Build Cells
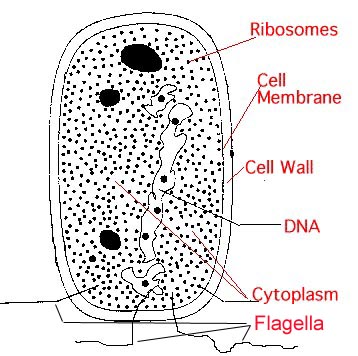 |
All this complex chemistry will proceed more easily if it is walled
off from interference. The first cells must have developed for this reason - thin
molecular walls that kept the "outside" out. The thin walls of the cells turned
out to have many other uses for regulating chemical reactions, as more sophisticated life
forms evolved
|
4.) Make Food
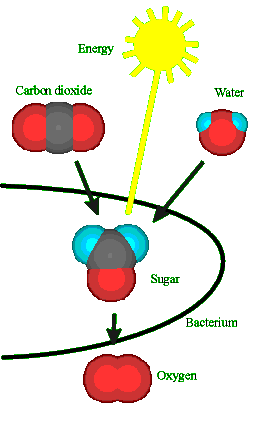 |
Although the very first life forms must have derived energy by
fermentation of organic chemicals, a huge advance came when higher-grade energy sources -
sugars - could be manufactured from the energy of the sun, photosynthesis. http://www.historyoftheuniverse.com/photosyn.html The cyanobacteria that make stromatolites are examples of the products of these four steps; they have cells containing DNA for reproduction and making energy by means of photosynthesis. |
Errors are the Key!
Mathematically, the number of generations needed for an advantage in survival of "s" to sweep through a colony of "N" inhabitants is (0.435/s) times log(2N). The early bacteria reproduced in about 30 minutes. Without worrying about the details, this formula shows that a modification that improves the chances of survival by 1 part in 1000 will sweep through a colony of a billion of them in about two years! (see "Endless Forms Most Beautiful" by Sean Carroll). Thus, "survival of the fittest" provided a powerful impetus for them to become ever more capable of surviving.
And survive they did!
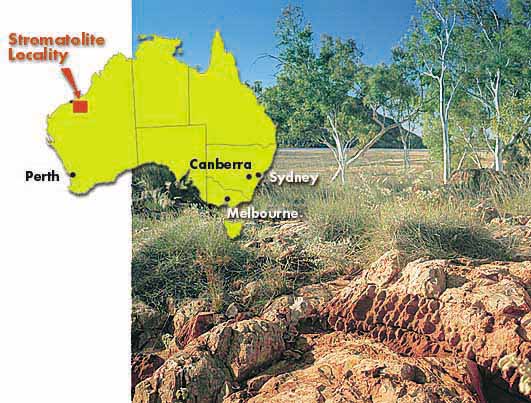 |
The cyanobacteria grew in vast colonies called stromatolites. At a site in NW Australia (left), a bed of
3.5 billion year old stromatolite fossils has been discovered (below left). (W.
Aust. Petroleum, http://www.dme.wa.gov.au/ancientfossils/)
|
 |
(W. Aust. Petroleum, http://www.dme.wa.gov.au/ancientfossils/) |
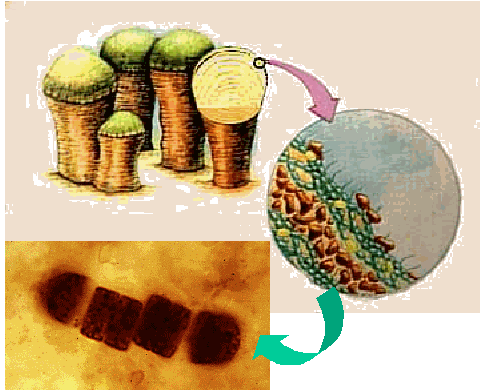 |
Stromatolites are an organization of the primitive cyanobacteria (lower
left) into large-scale structures that might have been analogous to coral reefs or
"pond scum" |
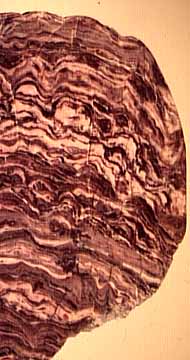 |
 |
Fossil stromatolites can be identified through a characteristic layering that results from the growth patterns of their constituent bacteria.(Worldwide Museum Nat. Hist., http://www.wmnh.com/wmel0000.htm B. G. Sutton http://www.lakeneosho.org/More7.html) |
|
|
|
| Here is how they may have
looked growing on the shore of an early ocean on the primitive earth.Artist's
concept (painting by Peter Sawyer,
|
There are still surviving living beds of these most primitive life forms in Australia and a few other places.(from J. Vicens http://www-ecpm.u-strasbg.fr/Actu/A-curious/a-c-stromato.html) |
Does this chain of events leading to primitive life occur often? Or is
life on earth a rare phenomenon? When we think of finding life on other worlds, we are
more interested in advanced life than in bacteria![]()
Search for Life on MarsThe question of how hard it is for life to form would be answered -- that it forms
easily -- if we could find life that formed somewhere else. Hence the great interest
(besides science fiction) in whether there is or ever was life on Mars. |
|
Life on Mars has held a fascination for us for many years
The Viking Mission in 1976 placed two landers on Mars.
| A picture sent back by a Viking lander showing its arm, which scooped up soil for testing: |  |
 |
 |
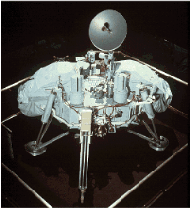
Picture of a Viking lander before launch (far left) Another view of the terrain near a Viking lander -- note how rocky it is! (left) http://www.tsgc.utexas.edu/images/planets/mars/vikinglander1-2.html |
Viking performed the following searches for life:
In the end, all the experiments gave no evidence for life.
A series of other Mars landers have carried out ever more sophisticated experiments (most recently by the Phoenix mission that landed in 2007), but they have all come up empty. But this evidence refers to now. Could Mars have had life in the past?
-- Recall geologic evidence for running water on Mars early in its history (e.g. ~ 3 billion years ago) and the Phoenix discovery of water just under the surface even now.
Mars is the most accessible place other than Earth where life may have formed; expect continued reports!
Test your understanding before going on![]()